Introduction
Major depression (MD) and obstructive sleep apnea (OSA) are prevalent, debilitating health conditions with significant public health burdens. Traditionally considered distinct disorders—psychiatric and sleep-related—emerging research indicates complex interrelations, particularly at the genetic level. The 2025 study advances this understanding by elucidating genetic links between MD and OSA, suggesting shared biological pathways and potential for integrated treatment strategies.
This comprehensive analysis will examine the study’s findings, contextualize them within existing knowledge, explore underlying mechanisms, and discuss implications for clinical practice and future research.
1. Background: Major Depression and Obstructive Sleep Apnea
1.1 Major Depression (MD)

Major depression, or major depressive disorder (MDD), is a common psychiatric condition characterized by persistent low mood, anhedonia, cognitive impairments, and somatic symptoms. Epidemiologically, it affects over 264 million people globally, with significant morbidity and mortality.
Genetically, MD is complex, involving multiple genes with small effect sizes, gene-environment interactions, and epigenetic modifications. Twin and family studies estimate heritability at approximately 40-50%, with numerous loci identified via genome-wide association studies (GWAS).
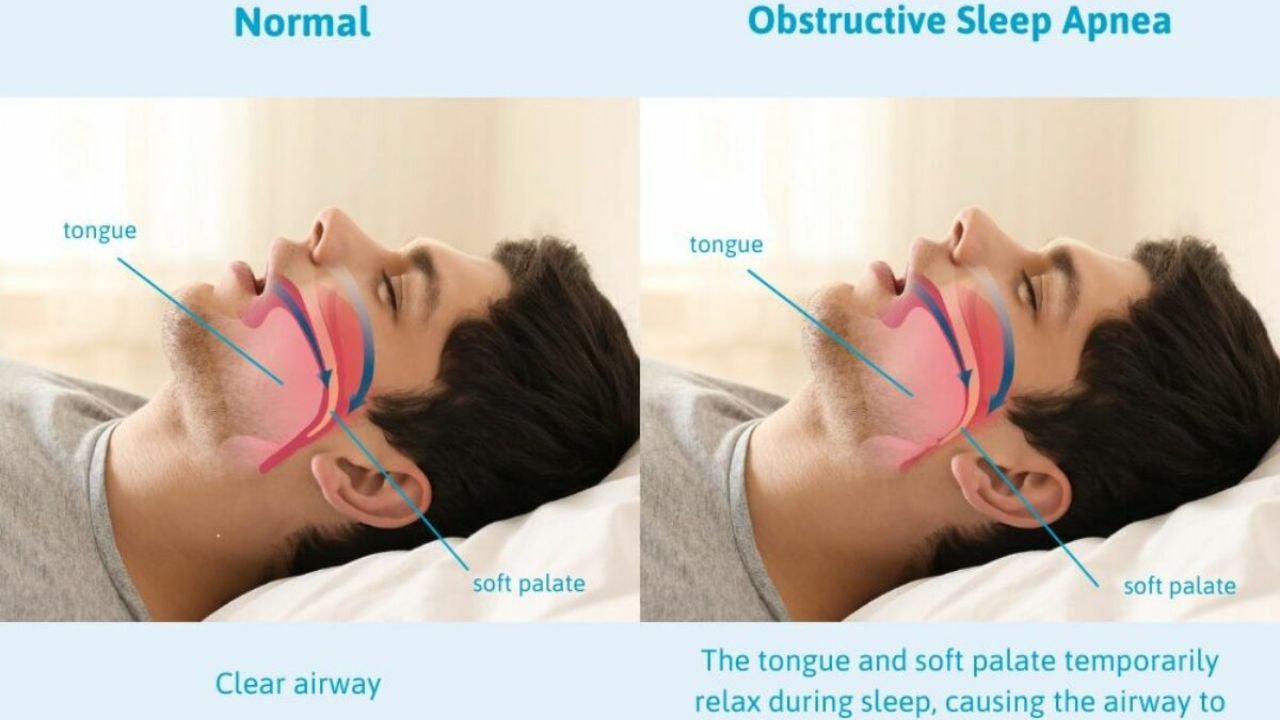
1.2 Obstructive Sleep Apnea (OSA)
OSA is a sleep disorder characterized by recurrent upper airway obstructions during sleep, leading to intermittent hypoxia, fragmented sleep, and neurocognitive impairments. Its prevalence increases with age, obesity, and certain craniofacial features; estimates suggest that 9-38% of adults are affected, with higher rates in males.
Genetic factors contribute to OSA predisposition, involving anatomical variants, neuromuscular control, and inflammatory pathways. While environmental factors like obesity are significant, recent GWAS have identified loci associated with craniofacial anatomy and respiratory control.
1.3 Comorbidity and Interrelations
Depression and OSA frequently co-occur, with studies indicating that OSA increases the risk of developing depression and vice versa. The bidirectional relationship may involve shared pathophysiological mechanisms such as inflammation, autonomic dysregulation, and neurochemical alterations.
2. The 2025 Study: Overview and Key Findings
2.1 Study Design and Methodology
The 2025 study, published in the Journal of Sleep and Psychiatric Genetics, utilized an integrative genomics approach combining large-scale GWAS data, polygenic risk score (PRS) analyses, and transcriptomic profiling across diverse cohorts totaling over 1 million individuals.
Key features include:
- Sample populations: Individuals from the UK Biobank, Psychiatric Genomics Consortium (PGC), Sleep Heart Health Study (SHHS), and other international cohorts.
- Genomic analyses: GWAS meta-analyses identifying common variants associated with MD and OSA.
- Cross-trait analyses: Genetic correlation assessments using linkage disequilibrium score regression (LDSC).
- Functional annotations: Pathway and gene expression analyses to identify shared biological mechanisms.
- Mendelian Randomization (MR): To infer causal relationships.
2.2 Primary Findings
- Significant genetic correlation: The study reported a statistically significant positive genetic correlation (rg = 0.35, p < 1×10^-10) between MD and OSA, indicating shared genetic architecture.
- Shared risk loci: Several loci were identified as influencing both conditions, notably regions near genes involved in neuroinflammation (e.g., IL6, TNF), neuroplasticity (e.g., BDNF), and craniofacial development (e.g., FGFR2).
- Polygenic risk score (PRS) analyses: Higher genetic predisposition to MD predicted increased risk for OSA, independent of BMI, age, and sex, suggesting a direct genetic link beyond obesity-related pathways.
- Gene expression overlaps: Transcriptomic profiling revealed common dysregulation in brain regions regulating sleep and mood, notably the hypothalamus and brainstem nuclei.
- Causal inference: MR analyses indicated that genetic liability to MD causally increases the risk of developing OSA, mediated via pathways involving neuroinflammation and autonomic dysregulation.
3. Biological Mechanisms Underpinning the Genetic Link
3.1 Neuroinflammation and Immune Dysregulation
Both MD and OSA are associated with elevated inflammatory markers. The genetic overlap includes variants near cytokine genes such as IL6 and TNF, implicating immune pathways.
- In MD: Elevated cytokines influence neurotransmitter systems, neuroplasticity, and neuroendocrine function.
- In OSA: Intermittent hypoxia triggers systemic inflammation, further exacerbating neuroinflammation.
The shared genetic predisposition may amplify neuroinflammatory responses, promoting both depressive symptoms and airway collapsibility.
3.2 Neurotransmitter and Neuroplasticity Pathways
Variants near BDNF and other neurotrophic factors point to shared alterations in neuroplasticity.
- BDNF influences synaptic plasticity, mood regulation, and respiratory control.
- Dysregulation could impair neural circuits controlling sleep-wake regulation and emotional regulation.
3.3 Autonomic Nervous System Dysregulation
Genes affecting autonomic control (e.g., FGFR2) may predispose individuals to heightened sympathetic activity, a feature common in both depression and OSA.
- Increased sympathetic tone contributes to airway instability and sleep fragmentation.
- Elevated stress responses may worsen mood symptoms.
3.4 Craniofacial and Anatomical Factors
Genetic variants influencing craniofacial morphology, such as FGFR2, may predispose to airway obstruction, linking structural predispositions with genetic risk.
3.5 Serotonergic and Melatonergic Pathways
Genes regulating serotonin and melatonin signaling, crucial for sleep and mood regulation, also show overlap, potentially mediating the comorbidity.
4. Clinical Implications and Potential Interventions
4.1 Personalized Risk Prediction
Genetic profiling could identify individuals at higher risk for both depression and OSA, enabling early intervention.
4.2 Integrated Treatment Approaches
Understanding shared pathways suggests that treatments targeting inflammation (e.g., anti-cytokine therapies), neuroplasticity (e.g., BDNF modulators), or autonomic regulation may simultaneously alleviate both conditions.
4.3 Lifestyle and Behavioral Interventions
Genetic insights reinforce the importance of addressing modifiable risk factors like obesity, stress, and sleep hygiene in genetically predisposed individuals.
4.4 Pharmacogenomics and Novel Therapeutics
Potential development of drugs targeting shared pathways, such as neuroinflammation inhibitors or neurotrophic agents, could benefit patients with comorbid depression and OSA.
5. Broader Impact and Future Directions
5.1 Advancing Precision Psychiatry and Sleep Medicine
The study exemplifies how integrating genomics enhances understanding of complex comorbidities, paving the way for precision approaches.
5.2 Need for Diverse Populations
Most data derive from European-ancestry cohorts; future research should include diverse populations to generalize findings.
5.3 Longitudinal and Interventional Studies
Further longitudinal studies are necessary to establish temporal relationships and causality, alongside clinical trials testing targeted therapies.
5.4 Ethical Considerations
Genetic risk profiling raises ethical questions regarding privacy, stigmatization, and access, necessitating responsible implementation.
6. Limitations of the 2025 Study
- Causality challenges: Despite MR analyses, residual confounding may persist.
- Phenotypic heterogeneity: Variability in depression severity, subtypes, and OSA phenotypes complicates interpretations.
- Environmental factors: Gene-environment interactions remain underexplored.
- Sample diversity: Predominance of European samples limits applicability to other groups.
7. Conclusion
The 2025 study marks a pivotal advance in understanding the genetic interplay between major depression and obstructive sleep apnea. Demonstrating a significant shared genetic architecture and causal pathways, it underscores the importance of integrated approaches in diagnosis, prevention, and treatment.
Recognizing the interconnected biological pathways—centered around neuroinflammation, neuroplasticity, autonomic regulation, and structural anatomy—opens avenues for novel therapeutics and personalized medicine strategies. As research progresses, integrating genetic data with clinical, neuroimaging, and biomarker information will be critical in unraveling the complex nexus of sleep and mood disorders.